NOTE:
12 AUGUST 2004 | DuPont is continuing to make the same excuses to the Environmental Protection Agency (EPA) on their violations of federal law. This analysis by EWG of DuPont's 2003 response to EPA's original inquiry still applies, since their defense has not changed, even after EPA announced it will formally take action against DuPont for violating section 8(e) of the Toxic Substances Control Act.
ORIGINAL CONTENT:
EWG letter to EPA concerning DuPont's response to EPA's inquiry of possible TSCA Section 8(e) substantial risk reporting violations
August 15, 2003
Mr. Richard H. Hefter, Chief
High Production Volume Chemicals Branch
U.S. Environmental Protection Agency
Office of Pollution Prevention and Toxics
1200 Pennsylvania Avenue, NW
Washington, DC 20460-0001
Re: DuPonts failure to submit key health studies under the requirements of TSCA 8(e), 15 U.S.C. § 2607(e).
Dear Mr. Hefter:
In a letter dated April 11, 2003 the Environmental Working Group (EWG) asked the Environmental Protection Agency (EPA or "the Agency") to investigate apparent violations of reporting requirements under Section 8(e) of the Toxic Substances Control Act (TSCA), 15 U.S.C. § 2607(e) (Section 8(e)), by a leading manufacturer and user of perfluorooctanoic acid (PFOA, C-8, APFO, FC-143), DuPont. The reporting violations involve the withholding of key studies for a period of more than 20 years. Had these study results been provided to the EPA as required by law, evidence of serious risks posed by PFOA would have become apparent to the Agency decades earlier than they ultimately were.
Specifically, EWG asked EPA to investigate DuPont's failure to notify EPA of a 1981 finding of birth defects in two of seven children born to PFOA-exposed female workers and a 1984 finding of PFOA (also called C-8 and FC-143) contamination in public drinking water in Little Hocking, Ohio [Read April 11, 2003 letter from EWG to EPA].
EWG has reviewed DuPont's response to the Agency in this matter, EPA's published Section 8(e) reporting requirements, additional documents recently made public relating to birth defects in PFOA-exposed female employees in DuPont's Washington Works plant in Parkersburg, West Virginia, and the company's internal response to this problem at that time. This new evidence provides substantial additional support that DuPont violated Section 8(e) of TSCA by failing to report the 1981 finding of serious and rare birth defects of the eye in babies born to 2 of 7 PFOA-exposed female workers. DuPont did not report these findings to the EPA at that time, and in fact has not officially reported these birth defects to the EPA even now. This is a clear violation of Section 8(e) of TSCA. According to TSCA Section 8(e) reporting requirements that applied to DuPont in 1981 and are still in effect today:
- A company is required to report to EPA any instance, even a single instance, of birth defects if a chemical is strongly implicated [Excerpt of 1978 notice | March 16, 1978 TSCA notice][Excerpt of 2003 notice | June 3, 2003 TSCA notice]. Internal company documents show that in DuPont's view, PFOA (C-8) was strongly, if not exclusively, implicated as the most likely cause of birth defects of the eye in children born to the companys employees. In March 1981, DuPont learned from 3M that PFOA had been linked to birth defects of the eye in studies on laboratory animals. Within two weeks, in response to this finding, DuPont announced to its workers that "all female employees will be removed from areas where there is potential for exposure to C-8 and loaned immediately to other divisions." Company documents show that by April, DuPont knew of eye defects among two children born to five female employees, and that the company considered this incidence rate to be 100 times higher than the national average. Additional documents from April 1981 indicated that DuPont found the birth defects a matter of significant concern, that the company was advising any female with PFOA exposures above background to consult her doctor before becoming pregnant, and that DuPont would conduct further studies designed to answer a single question does PFOA [C-8] exposure cause abnormal children. EPA's guidance on this issue has not changed and still requires companies to report a single birth defect if one, or a few chemicals, is strongly implicated [Excerpt | June 3, 2003 TSCA notice].
- A company is required to report adverse health findings if the information reasonably supports a conclusion of substantial risk, if the company can reliably ascribe the effect to the chemical, or if the chemical is strongly implicated in the finding. As noted, as a result of both the laboratory studies and its findings of birth defects among children of its employees, DuPont moved female employees out of PFOA production areas, initiated further pregnancy outcome studies for employees exposed to PFOA, advised women exposed to PFOA to consult with their physicians before becoming pregnant, and initiated reviews and further animal studies of PFOA that would not be completed until well after the 15-day reporting deadline under TSCA Section 8(e). Only PFOA, and no other chemical, was implicated by DuPont as potentially related to the birth defects. Each step undertaken and documented by DuPont in the months following its initial birth defect study show that the company did, in fact, interpret the available information as meeting the burden of reasonably supporting, reliably ascribing, and strongly implicating conclusions that PFOA exposures could be linked to the birth defects.
- A company is not to delay reporting until it obtains conclusive information that a substantial risk exists, but must immediately report any evidence that reasonably supports a conclusion of substantial risk [Excerpt | March 16, 1978 TSCA notice]. EPAs interpretation of reporting requirements did not give DuPont the luxury of conducting follow-up studies to confirm the initial findings, but instead required immediate reporting of the birth defects. The 15-day deadline for reporting passed no later than June 4, 1981. DuPont would also have violated current reporting timeframe requirements, which were changed from 15 business days to 30 calender days on June 3, 2003. To this date, DuPont has not voluntarily informed EPA of the findings, even after EPA raised serious concerns regarding PFOA toxicity.
DuPonts 1981 finding of PFOA contamination in the public drinking water in Little Hocking, Ohio met the following EPA reporting requirements:
- A company is required to report finding widespread and previously unsuspected distribution in environment media [Excerpt | March 16, 1978 TSCA notice]. DuPonts finding of PFOA (C-8) contamination in a community drinking water supply indicated previously unsuspected, widespread exposure to several thousands of people. The reporting requirements do not include the option for a company to opt out of the submission requirement based on a company's assessment of risk posed by the newly-discovered contamination. Notably, DuPont lacked the data at the time to assess human health risk, having failed to establish a safe dose in the single long-term toxicity study available. As justification for failing to report under Section 8(e), DuPont cites proposed EPA guidance released nine years after their drinking water findings, well beyond the 15-day reporting deadline. The latest 2003 guidance would also require DuPont to submit the drinking water findings to EPA [Excerpt | June 3, 2003 TSCA notice].
- A company is required to report a finding that reasonably supports the conclusion that a chemical presents a substantial risk of injury to health [Excerpt | March 16, 1978 TSCA notice]. When DuPont discovered PFOA contamination in a public drinking water supply, the only long-term laboratory study available on health effects from PFOA exposure had shown adverse effects at the lowest dose tested, with female rats showing statistically significant increases in mammary gland fibroadenomas, ataxia and ovarian tubular hyperplasia. In justifying their failure to report finding PFOA in drinking water, DuPont has argued that the levels found did not present any risk of injury. Yet at the time of the tests, DuPont was aware of PFOAs persistence in the environment, and its long human half-life, characteristics that increase exposure concerns. In addition, the only laboratory test relevant to long-term exposures such as those faced by the community drinking contaminated water had failed to find a safe dose. The most scientifically defensible position available to DuPont in 1984 would have been to find that the levels of PFOA in drinking water could have posed a substantial risk to human health.
Further details and supporting documents on DuPonts historic interpretation of each of the studies currently under investigation by EPA are presented below.
A. BIRTH DEFECTS
On March 16, 1978, EPA published a statement of interpretation and enforcement policy regarding what constitutes a Toxic Substances Control Act (TSCA) section 8(e) substantial risk notification [1]. Guidance for what constitutes a substantial risk is essentially unchanged over the past 25 years. In the 1978 guidance document, EPA states that findings of birth defects, if one or a few chemicals are strongly implicated, should be reported as a TSCA 8(e) "substantial risk" notice.
V. What Constitutes Substantial Risks
The Agency considers effects for which substantial-risk information must be reported to include the following:
(a) Human health effects (1) Any instance of cancer, birth defects, mutagenicity, death, or serious or prolonged incapacitation, including the loss of or inability to use a normal bodily function with a consequent relatively serious impairment of normal activities, if one (or a few) chemicals(s) is strongly implicated. [Emphasis added]
Contrary to assertions made by DuPont in their response to EPA [DuPont June 20, 2003 response to EPA], a finding of significant risk does not mean that TSCA 8(e) requirements only apply if a quantitative risk assessment shows that a substance presents an unacceptable, or "substantial" level of risk to employees. Rather, significant risk reporting criteria, in effect in 1981 and still in effect, apply whenever a substance is "strongly implicated" in any instance of a specified adverse health effect, in this case a birth defect.
DuPont violated this 1978 Section 8(e) regulation, as shown by the following timeline of key events that occurred between March 20 and May 14, 1981. The chronology provides compelling evidence, in the form of internal company documents, that DuPont scientists strongly, if not exclusively, implicated PFOA (C-8) as the cause of birth defects of the eye in children born to female employees exposed to PFOA (C-8), and that the company should have submitted the human birth defect findings to EPA as a TSCA 8(e) substantial risk notice. Much of what we present below is taken from previously undisclosed DuPont correspondence recently made public through court proceedings. This information, along with supporting documents, has been presented to Richard H. Hefter, Chief of EPA's High Production Volume Chemicals Branch in a July 3, 2003 letter from Rob Bilott, an attorney at Taft, Stettinius & Hollister, LLP. This letter is also available in EPA's administrative record for PFOA (AR226-1372) [2].
March 20, 1981. 3M informed both EPA, via a TSCA Section 8(e) notice, and DuPont that eye birth defects were observed in a PFOA, also called T-2998CoC, oral rangefinder birth defect ("teratology") study. In this study, fifteen to sixteen fetuses (four fetuses from four pregnant rats) were examined from the high and low dose groups. One hundred percent of fetuses examined had eye defects [Excerpt | Full Document]. The eye defects, described later as "scarring", involved one or more of the following: large lens clefts, dark streak running one-half to three-quarters of the way through the lens or disorganized lens fibers. 3M also noted that similar lens abnormalities had been observed in previous rat teratology studies with two other compounds chemically related to PFOA [3].
March 25, 1981. In a memo marked "personal and confidential" from DuPont Medical Director, Dr. Bruce Karrh, to C. De Martino summarizing the 3M rat eye defect study, Dr. Karrh stated that "If the 3M study is valid, women of child-bearing potential will probably be excluded from jobs where there is potential for exposure to C-8 [PFOA] compounds, at least until a no-effect level is determined" [View document] [4].
March 27, 1981. An April 1, 1981 memo from J.W. Raines to R.L. Richards states that two Haskell Lab scientists, Dr. R.E. Staples, a teratologist, and Taison Chiu, a pathologist, visited 3M on March 27, 1981 to review the C-8 (PFOA) range finder study results. They concluded "that the study was valid and that the observed fetus eye changes were due to the C-8" [View document] [5].
March 31, 1981. DuPont notified its employees in an "Employee Communication" that all female workers would be removed from jobs "where there is potential for exposure to C-8 [PFOA]" at DuPont's Washington Works plant [Excerpt | Full document] [6].
April 1, 1981. DuPont began blood sampling of female employees from the Teflon area with the purpose of quantifying PFOA levels [View document] [7].
April 6, 1981. DuPont Medical Director, Dr. Bruce Karrh, sent a "personal and confidential" memo to Carl De Martino and other DuPont medical staff stating that DuPont Medical Division should move ahead with a delayed pregnancy outcome study "among C-8 [PFOA] exposed male and female employees" because "recently obtained information indicates there may be a need to do such a study" [View document] [8]. An earlier memo, dated April 2, 1981, makes it clear that the focus of the pregnancy outcome study was PFOA. In Dr. Karrh's own words, "pregnancy outcomes can be studied to answer a single question - does C-8 [PFOA] exposure cause abnormal children" [View document] [9]. No other chemicals are mentioned.
April 8, 1981. On Wednesday April 8, 1981, both the New York Times and Wall Street Journal report on the preliminary findings from the FC-143 (PFOA) rat teratology study conducted by 3M. The articles also note that women at the DuPont plant near Parkersburg, West Virginia were transferred to other jobs in light of the preliminary findings and that additional laboratory tests were planned. At no point do the articles mention that birth defects were noted in two babies recently born to women at the plant who worked with PFOA. Therefore, the New York Times and Wall Street Journal articles do not fulfill DuPont's TSCA Section 8(e) submission requirements regarding the reports of two birth defects in babies born to women exposed to PFOA at the plant [Excerpt | Full document] [10].
April 9, 1981. In additional communication materials prepared for Washington Works employees, DuPont states:
We do know of two women who worked in this area before and during pregnancy whose children reportedly had defects detected at birth. We became aware of this information after 3M notified us of the animal study. We do not know whether there is a relationship. We are investigating this matter further, and we are considering additional studies.
Some employees have asked what advice we have for female employees of childbearing potential who have been exposed to FC-143 [C-8] about becoming pregnant. Until we have additional information about the potential effects of FC-143 on the human fetus, we think this is a matter of sufficient concern that, as a precaution, a female who has an organic fluorine level above background level should consult with her personal physician prior to contemplating pregnancy. We will provide all information we have on FC-143 to employees' personal physicians [11].
April 13, 1981. DuPont Medical Division staff, epidemiologist Dr. William Fayerweather, circulated a "Study of Pregnancy Outcome in Washington Works Employees: Research proposal" that stated that the objectives of the study are to determine whether "a. Pregnancy outcome among female Washington Works employees is causally related to their occupational exposure to C-8 [PFOA]" and whether "b. Pregnancy outcome among wives of Washington Works employees is causally related to their husbands' exposure to C-8" [Excerpt | Full document] [12].
This same research proposal indicated that a finding of two malformations in either five or ten exposed live births would be significantly higher than both a national rate of two craniofacial malformations per 1000 and a plant with zero birth defects per 50 non-exposed births [Excerpt | Full document].
April 28, 1981. A memo from Dr. Burford Culpepper to H.G. Smyth noted that Haskell laboratories was initiating animal studies and results were not expected for 60 days [View document] [13].
May 4, 1981. A DuPont "Time Line for C-8 Control Program" document indicates that additional toxicity testing is started at Haskell Laboratory [Excerpt | Full document] [7].
May 14, 1981. Documents labeled "Personal and Confidential, revised 5/14/81" indicate that DuPont had measured PFOA (C-8) in the blood of five women who had given birth to live babies during the past several years. Two of the five babies had birth defects involving the eye. Two women, one who gave birth to a normal child and another who gave birth to a baby with an "unconfirmed eye and tear duct defect", had PFOA blood levels above DuPont's interim blood level standard of 0 to 0.4 ppm total organic fluoride [View document] [14].
The presence of birth defects in two of the five births would appear to be an important elevation in the birth defect rate since DuPont had already determined that two malformations in ten births would be a statistically significant increase compared to either national or plant rates.
A subsequent undated update, reflecting a birth in July and one in August of 1981, shows that two of seven live births resulted in babies with eye defects [View document] [15].
June 4, 1981. Fifteen business days after the May 14, 1981 document, DuPont failed to inform EPA of the birth defects noted in two of these employees. To this date, DuPont has never voluntarily informed EPA of the findings, even after EPA raised serious concerns regarding PFOA toxicity.
IV. Requirement that a Person "Immediately Inform" the Administrator
With the exception of information on emergency incidents of environmental contamination (see Part V(c)] a person has "immediately informed" the Administrator if information is received by EPA not later than the 15th working day after the date the person obtained such information [1].
[March 16, 1978 TSCA notice: Excerpt | Full document]
July 22, 1981. A document describing the status of the pregnancy outcome study states that the "study was 'on hold' until further notice." The DuPont document does not say why the study was put on hold. Notably, the pregnancy outcome study is suspended before results of the follow-up teratology studies appear in memos [View document] [16].
September 15, 1981. An internal DuPont memo states that results of 3M repeat study not expected until October 1981 [Excerpt | Full document] [15].
December 15, 1981. In a memo from R.J. Burger to Fluoropolymer Supervision regarding follow-up rat teratology studies conducted by DuPont and 3M, Burger states:
Thus far, based on our review of the results of these further studies, it does not seem that the observed effects in the eyes of the unborn rats were due to C-8 [PFOA]. Also in the new studies, rat pups delivered by C-8 exposed females showed no eye defects. Rather, it is believed that in the original studies, 3M's technique for the very difficult job of preparing the fetal eye tissue for microscopic examination resulted in the alterations noted [17].
February 4, 1982. In a memo dated February 4, 1982, B.C. McKusick summarizes for M.A. Smook teratology data presented at a February 3, 1982 meeting with 3M in Chicago. None of the four teratology studies, two conducted by 3M and two conducted by DuPont, report eye defects.
Lamprecht [E. Lamprecht, 3M pathologist] described two negative oral teratology studies with C-8 [PFOA], one in rats, one in rabbits. Griffith gave us a copy of the rat report. The rabbit report is circulating for approval and should be in official form in about two weeks.
Staples [R.E. Staples, DuPont teratologist] described the two Haskell teratology studies in rats, one feeding and one inhalation study. Both are negative [18].
March 1, 1982. On March 1, 1982, an employee communication document states that females of child-bearing age can move back into areas with potential C-8 (PFOA) exposure because follow-up rat teratology studies did not reveal birth defects [View document] [19].
June 20, 2003. In response to an EPA inquiry into potential 8(e) violations, DuPont stated "DuPont recently found that the umbilical cord blood of the child of the fourth employee on the list was tested as well. The level reported was 0.43 ppm..." [Excerpt | Full document] [10]. In 1981, women with a total organic fluoride blood level greater than 0.4 ppm were removed from the fluoropolymer duties [View document] [13].
In addition to the rat eye defect finding, other PFOA data were available prior to the 1981 human birth defect discovery that would support a TSCA 8(e) submission from DuPont regarding the presence of the chemical in female workers and babies. For instance, by 1981 DuPont was aware that PFOA was persistent in people [Excerpt | Full document] [6], characteristics that increase exposure concerns for a chemical.
Regardless of DuPont's ultimate conclusion about the potential role of PFOA in the rat teratology study over the next year, DuPont was obligated to report the human birth defect findings at least by May or June 1981. The only data available during this period "reasonably supports" the conclusion that PFOA could have caused the eye defects noted in two babies born to workers exposed to PFOA. EPA guidelines in effect at that time clearly indicate that 8(e) notices should occur when there is "reasonable support" and not "conclusive information" regarding substantial risk. These guidelines still apply and have not been changed during the past 25 years. Negative follow-up rat and rabbit studies do not diminish the importance of finding birth defects in two of seven live births, an elevation in incidence that DuPont considered statistically significant.
"A person is not to delay reporting until he obtains conclusive information that a substantial risk exists, but is to immediately report any evidence which 'reasonably supports' that conclusion. Such evidence will generally not be conclusive as to the substantiality of the risk; it should, however, reliably ascribe the effect to the chemical."
Comment 6: Section 8(e) obligations are incurred upon obtaining conclusory substantial-risk information.
Response: The Agency disagrees, and considers that "reasonable support" of a conclusion of substantial risk is not identical to the conclusion itself. The former typically occurs, and must be reported, at an earlier stage [Emphasis added] [1].
[March 16, 1978 TSCA notice: Excerpt | Full document]
B. PFOA IN DRINKING WATER
Between March and June 1984, DuPont tested for, and found, PFOA in tap water taken from a store in Little Hocking at levels of 0.8 and 0.6 ppb. However, DuPont failed to inform U.S. EPA of the finding, and did not inform the Little Hocking Water Association of this finding until seventeen years later in 2001 [see Little Hocking Water Statement], when it was revealed during the course of establishing a consent order between DuPont and the West Virginia Department of Environmental Protection.
The 1984 finding of PFOA in drinking water indicates widespread exposure to communities near DuPont's Washington Works Plant in Parkersburg, WV. Guidance provided by the EPA in 1978 as to what constitutes an TSCA section 8(e) substantial risk indicate that DuPont committed a 8(e) violation by failing to inform EPA of the presence of PFOA in drinking water.
V. What Constitutes Substantial Risks
(a) Environmental effects - (1) Widespread and previously unsuspected distribution in environmental media, as indicated in studies (excluding materials contained within appropriate disposal facilities) [1].
[March 16, 1978 TSCA notice: Excerpt | Full document]
DuPont has argued that the presence of PFOA at the levels detected does not constitute a "substantial risk" and therefore was not subject to TSCA Section 8(e) reporting. This was not the burden of proof in place in 1984 when the water tests were performed. DuPont did, in fact, find the "widespread and previously unsuspected distribution in environmental media" that should have triggered reporting. Current TSCA Section 8(e) guidelines would also require DuPont to report the drinking water findings.
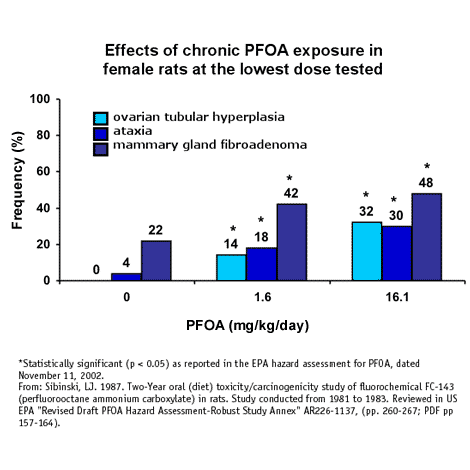
But even from the perspective of human health risk, the findings should have triggered reporting. Prior to detecting PFOA in drinking water, neither DuPont nor 3M had found a dose of PFOA that did not cause adverse health effects in animals exposed for more than three months [20]. Most notably, a two-year cancer study completed in 1983 found significant effects in female rats at the lowest dose tested. At 1.6 mg/kg/day, PFOA caused significantly increased incidence of muscle incoordination, ovarian tubular hyperplasia and mammary gland fibroadenomas (Figure 1) [21]. In fact, to this day a "no observable effect level" (NOEL) has yet to be determined in laboratory animals exposed to PFOA in chronic exposure studies lasting for more than 25 percent of a lifetime.
In the absence of a chronic "no observable effect level," DuPont is in a difficult position to argue that a lifetime exposure to PFOA in drinking water at any level does not potentially constitute a substantial risk. In fact, DuPont used this same line of reasoning in April 1981 to describe one of the goals of a series of planned teratogenicity studies with respect to establishing an acceptable exposure limit for women of childbearing capacity:
Both DuPont and 3M plan to start full-scale teratogenicity studies promptly. A major goal will be to determine a dosage or exposure concentration of FC-143 [PFOA] that does not cause birth defects and to relate this dosage to blood levels of FC-143. Until we have these data, we have no good basis for setting an acceptable exposure limit (AEL) for women of childbearing capacity.
[Excerpt | Full document] [22]
In addition, DuPont was aware in 1984, as of 1978, in fact, that PFOA was persistent in both the environment and humans (i.e. had a long human half-life), which further elevates exposure concerns.
The EPA TSCA 8(e) substantial risk notification guidelines in effect in 1984 do not distinguish between a "safe" and "unsafe" level of distribution. Therefore, it is irrelevant that DuPont did not consider the level of PFOA detected in drinking water to be a "substantial risk". New TSCA 8(e) guidelines issued on June 3, 2003 also do not make a distinction for a "safe" or "unsafe" level of exposure for contaminants that do not have established EPA maximum contaminant levels (MCL) in drinking water, ambient water quality criteria for receiving bodies of water, and reference doses (RfD) or reference blood concentrations (RfC) [June 3, 2003 TSCA guidance: Excerpt | Full document][23]. PFOA is still an unregulated chemical and thus had no EPA established MCL, RfD or RfC in 1984. Moreover, DuPont did not establish an internal community exposure guideline (CEG) until 1987, three years after finding PFOA in drinking water [View document] [24]. Although DuPont notes that levels detected in drinking water do not exceed a controversial screening level established by the C-8 Assessment of Toxicity Team (CATT) under the consent order, the EPA has not endorsed the CATT level.
In summary, DuPont was obligated to report the Little Hocking drinking water finding on two counts one, that the finding met the reporting criteria for widespread and unsuspected contamination; and two, that the finding was consistent with an interpretation of "substantial risk" because available data at the time showed that PFOA was persistent and toxic at low levels. In the absence of a NOEL for laboratory animals, DuPont would appear to be in a weak position to argue that any level of PFOA detected in drinking water was not a "substantial risk."
1983. 3M's Riker laboratory completed a two-year chronic toxicity/cancer study for PFOA. Significant increases in mammary gland fibroadenomas and Leydig cell adenomas were noted in female and male rats. At the lowest dose tested, 1.6 mg/kg/day, female rats had statistically significant increases in mammary gland fibroadenomas, ataxia and ovarian tubular hyperplasia (Figure 1) [View EPA summary] [21, 25]. To date, a dose or concentration of PFOA has not been identified that does not cause serious toxicity in laboratory animals exposed for more than several months.
March to June 1984. DuPont tested Little Hocking water twice during this period from a store in Little Hocking: the first indicated that PFOA (C-8) was detected at 0.8 parts per billion (ppb); and the second found C-8 at the detection limit of 0.6 ppb [Excerpt | Full document] [26].
May 22, 1984 - On May 22, 1984, key DuPont staff met at company headquarters in Wilmington, Delaware to discuss PFOA (C-8), including the recent detection of C-8 in Little Hocking, OH and Lubeck, WV water systems. In a meeting summary memo marked "Personal and Confidential", written the next day by J.A. Schmid to T.M. Kemp, T.L Schrenk and R.E. Putnam, Schmid writes:
There was agreement that a departmental position needed to be developed concerning the continuation of work directed at elimination of C-8 exposures off plant as well as to our customers and the communities in which they operate.
There was consensus reached that the issue which will decide future action is one of corporate image, and corporate liability. Liability was further defined as the incremental liability from this point on if we do nothing as we are already liable for the past 32 years of operation. Corporate image discussion centered around the perceived diligence versus our policies if we elected to stop work.
Currently. None of the options developed are, from a fine powder bussiness [sic] standpoint, economicaly attractive and would essentially put the long term viability of this bussiness [sic] segment on the line. From a broader corporate viewpoint the costs are small.....Looking ahead. Legal and medical will most likely take a position of total elimination. They have no incentive to take any other position. The product group will take a position that the bussiness [sic] cannot afford it. The end result, in my opinion, will be that we eliminate all C-8 emmisions at our manufacturing sites in a way yet to be developed which does not economically penalize the bussiness [sic], and addresses the C-8 emission and exposures of our dispersion customers.
[Excerpt | Full document] [27]
DuPont now argues that the amount of PFOA found in Little Hocking drinking water in 1984 was not high enough to be considered a substantial risk [View DuPont's June 20, 2003 response to EPA], even though a long-term exposure animal study completed in 1983 failed to find a dose that did not cause significant toxicity to female rats (Figure 1). This meeting summary memo indicates that DuPont's medical and legal staff interpreted the drinking water results with a high level of concern. It makes little sense that DuPont would have conducted the initial tests, and follow-up tests over the next few years, if there were no concerns about human health risks to the community as claimed.
June 25, 1987. For the first time, DuPont develops an acceptable level for PFOA in community drinking water of 5 parts per billion (ppb). This level does not account for exposures to PFOA that might occur from other sources, such as the air [View document] [28].
March 13, 1987 and May 12, 1988 - DuPont tested for C8 in Mason's Village Market and the Ritenour home in Little Hocking, and did not find it above the test detection limit [Excerpt | Full document] [26].
November 7, 1988. DuPont tested for PFOA (C-8) in the Ritenour home in Little Hocking. The analysis report listed the result as: "contaminated sample, analysis not possible" [View document] [29].
May 4, 1989. DuPont tested again in the Ritenour home. The analysis report gave the result, again, as: "contaminated sample, analysis not possible." EWG found no evidence that follow-up tests were conducted [View document] [30].
September 19, 1991. A revised CEG for PFOA of 1 ppb is in effect. This level assumes that 20% of exposure to PFOA occurs through drinking water and 80% comes from the air [Excerpt | Full document] [26].
December 20, 2001. DuPont analyzes water from four Little Hocking drinking water production wells and finds PFOA levels of 1.84, 3.73, 0.86 and 7.69 ppb. Three of these values exceed DuPont's CEG of 1 ppb. The most recent samples taken from Little Hocking production wells, on February 26, 2003, had PFOA levels of 3.38, 3.62, 0.733, 6.93 and 7.16 ppb [Little Hocking Water Association PFOA test results].
To this date DuPont has not voluntarily informed EPA of the contamination found in Little Hocking's drinking water in 1984. Seventeen years later, after learning of PFOA contamination in tap water from the neighboring community of Lubeck, WV, Little Hocking Water Association officials petitioned WV DEP to test city water wells. Only then did the community learn of the contamination [Read the Little Hocking news release].
Given the nature and seriousness of the omissions, we recommend that the Agency levy the maximum allowable penalty under the law, a $25,000 fine per day to account for civil violations pursuant to 15 U.S.C § 2615 (a). We also ask that you investigate potential criminal violations for DuPont's knowing and willing failure to produce these studies, which would also subject the company to a maximum daily fine of $25,000 [Id. at § 2615 (b)].
Sincerely,
[signed]
Kenneth A. Cook President, Environmental Working Group
cc: Charles O. Holliday, Jr., Chairman & CEO, DuPont
Steve Johnson, EPA's Acting Deputy Administrator
Susan B. Hazen, EPA's Acting Assistant Administrator of the Office of Prevention, Pesticides, and Toxic Substances (OPPTS)
References
[1] Environmental Protection Agency (EPA). 1978. Toxic Substances Control Act: Statement of Interpretation and Enforcement Policy; Notification of Substantial Risk. Federal Register March 16, 1978 (Volume 43, Number 52): 11110-11116.
[2] Bilott, R. 2003. TSCA Section 8(e) reporting for PFOA. Taft, Stettinius & Hollister LLP (July 3, 2003). US EPA AR226-1372.
[3] 3M. 1981. Section 8(e) Toxic Substances Control Act (TSCA) Perfluoroalkane Carboxylic Acid and Corresponding Ammonium Carboxylates. March 20, 1981: US EPA AR226-1373.
[4] DuPont. 1981. Personal and confidential memo from Dr. Bruce W. Karrh to C. De Martino: Ammonium Perfluorooctanoate (FC-143) C-8 Compounds - March 25, 1981. US EPA AR226-1373.
[5] DuPont. 1981. Memo from J.W. Raines to R.L. Richards: Ammonium Perfluorooctanoate (C-8) Rangefinder Study - April 1, 1981. US EPA AR226-1377.
[6] DuPont. 1981. Memo from R.J. Burger to Supervision Through Division Superintendent - March 31, 1981. US EPA AR226-1378.
[7] DuPont. 1981. Attachment I: Time Line For C-8 Control Program (March 20, 1981 - August 4, 1981). US EPA AR226-1374.
[8] DuPont. 1981. Personal and confidential memo from Dr. Bruce W. Karrh to C. De Martino: Epidemiology Study -C-8 (FC-143) - April 6, 1981. US EPA AR226-1380.
[9] DuPont. 1981. Memo from Dr. Bruce W. Karrh to Carl De Martino: Epidemiology Study - C-8 (FC-143) - April 2, 1981. US EPA AR226-1379.
[10] DuPont. 2003. Letter from Dupont to Mr. Richard H. Hefter (Chief of the High Production Volume Chemicals Branch) regarding TSCA Section 8(e) reporting requirements - June 20, 2003.
[11] DuPont. 1981. Attachment V: Washington Works proposed communication to females who had worked in fluoropolymers area - April 9, 1981. US EPA AR226-1374.
[12] DuPont. 1981. Study of pregnancy outcome in Washington Works employees: research proposal by Dr. William E. Fayerweather - April 13, 1981. US EPA AR226-1381.
[13] DuPont. 1981. Memo from Dr. Burford W. Culpepper to H.G. Smyth - April 28, 1981. US EPA AR226-1385.
[14] DuPont. 1981. Personal and confidential memo from R.D. Ingalls to J.H. Todd: C-8 Program Status - May 26, 1981. US EPA AR226-1388.
[15] DuPont. 1981. Memo from Paul Thistleton to S. Takada: Proposed employee sampling program - September 15, 1981. US EPA AR226-1390.
[16] DuPont. 1981. Memo regarding objectives, study design and status of the pregnancy outcome study. US EPA AR226-1383.
[17] DuPont. 1981. Memo from R.J. Burger to Fluoropolymers Supervision: C-8 (FC-143) status report - December 15, 1981. US EPA AR226-1392.
[18] DuPont. 1982. Memo from Confidential memo from B.C. McKusick to M.A. Smook: Meeting with 3M on C-8 (FC-143) - February 4, 1982. US EPA AR226-1394.
[19] DuPont. 1981. C-8 (FC-143) Employee Communication - March 1, 1982. US EPA AR226-1395.
[20] Environmental Protection Agency (EPA). 2002. Revised draft hazard assessment of perfluorooctanoic acid and its salts, November 4, 2002. US EPA AR226-1136.
[21] Sibinski, LJ. 1987. Two-Year oral (diet) toxicity/carcinogenicity study of fluorochemical FC-143 (perfluorooctane ammonium carboxylate) in rats. Report prepared for 3M, St. Paul, Minnesota by Riker Laboratories Inc. Study No. 0281CR0012; 8EHQ-1087-0394, October 16, 1987 Reviewed in US EPA "Revised Draft PFOA Hazard Assessment-Robust Study Annex". US EPA AR226-1137, (pp. 260-267; PDF pp 157-164).
[22] DuPont. 1981. Personal and confidential memo from Drs. C.F. Reinhardt and B.W. Karrh to H.E. Serenbetz: FC-143 (Ammonium perfluorooctanoate; C-8 CAS-3825-26-1) - April 10, 1981. US EPA AR226-1388.
[23] Environmental Protection Agency (EPA). 2003. TSCA Section 8(e); Notification of Substantial Risk; Policy Clarification and Reporting Guidance. Federal Register June 3, 2003 (Volume 68, Number 106): 33129-33140.
[24] DuPont. 1981. Memo from H.A. Smith to G.L. Kennedy: Ammonium perfluorooctanoate (C-8) - June 12, 1987. US EPA AR226-1401.
[25] Environmental Protection Agency (EPA). 2002. Annex of robust study summaries from the revised draft hazard assessment of perfluorooctanoic acid and its salts, November 4, 2002. US EPA AR226-1137.
[26] DuPont. 1991. Memo from Terry Vandell to Walt Stewart: Meeting minutes of the on-site Washington Works meeting (September 11, 1991, 9:00 AM-11:00 AM) regarding the September 4, 1991 proposed C-8 Sampling Program - September 19, 1991. US EPA AR226-1406.
[27] DuPont. 1987. Memo from J.A. Schmid to T.M. Kemp, T.L. Schrenk and R.E. Putnam: C-8 Meeting Summary - May 23, 1984.
[28] DuPont. 1987. Memos from H.A. Smith to G.L. Kennedy: Ammonium perfluorooctanoate (C-8) - June 12, 1987 and from G.L. Kennedy to H.A. Smith US EPA AR226-1401 and 1402.
[29] DuPont. 1989. Interoffice memorandum from Anthony J. (Tony) Playtis to Roger J. Zipfel, John E. Crum, and Walter M. Stewart. Subject: Test Results - C8 in Water (January 30, 1989). US EPA AR226-1246 (attachment 28; PDF page 132).
[30] DuPont. 1989. Interoffice memorandum from Anthony J. (Tony) Playtis to William E. Crawley, John E. Crum, Walter M. Stewart, Bill Tice, and Roger J. Zipfel. Subject: Test Results - C8 in Water (June 30, 1989) US EPA AR226-1246 (attachment 33; PDF page 147).