NOVEMBER 17, 2004 — The Environmental Working Group (EWG) has uncovered evidence that DuPont is suppressing new study results that show significant health risks from their Teflon ingredient PFOA (C8) (Exhibit A, B). The results, reported below, show what appear to be the highest blood levels of the Teflon chemical ever found outside of the workplace in the United States (Exhibit A).
DuPont's apparent suppression of critical health information occurs in the midst of the company's legal battle with the Environmental Protection Agency (EPA) over their failure to disclose health risks from previous studies, with charges that carry a fine of up to $313 million.
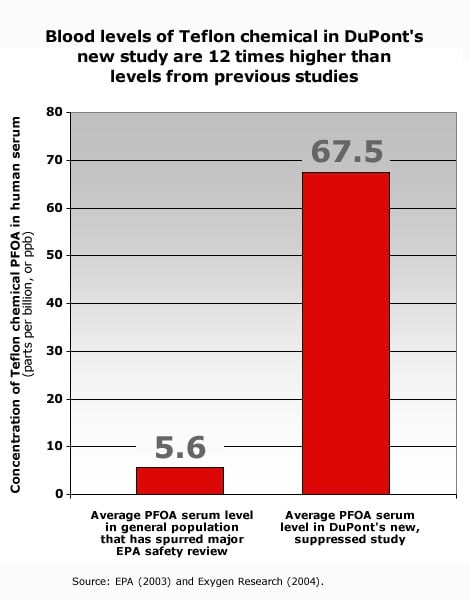
Today EWG is petitioning the federal government to conduct a full investigation of this apparent violation of federal reporting requirements for toxic chemicals under the Toxic Substances Control Act, 15 U.S.C. § 2607(e). DuPont's new, suppressed study obtained by EWG shows that DuPont found PFOA in the blood of people living near their West Virginia Teflon plant at levels 12 times higher than U.S. background blood levels of the pollutant (Exhibit A, B).
While the Environmental Protection Agency conducts what the Agency has termed "the most significant scientific assessment ever conducted" because of their concerns over human exposures, DuPont has apparently chosen to suppress data that could be critical in the Agency's efforts to protect public health.
In a risk assessment published in April 2003, EPA found that human blood levels are within a factor of seven from "no effect" levels in laboratory studies. The new, suppressed study drives this so-called margin of exposure, already dramatically lower than the value of 100 typically required by the Agency, down to a mere factor of three.
|
EPA's formal complaint against the company, filed last July in response to an EWG petition, charges DuPont with three counts of failing to report company information on substantial health risks from other company studies regarding the Teflon chemical, as required under TSCA Section 8(e), 15 U.S.C. § 2607(e). According to EPA's enforcement official, the complaint was filed with an intention "to send a message to DuPont and everyone else [that] this type of information must be provided" so officials "can make valid assessments of the risks posed by various substances." Even as DuPont prepares for a December 16th hearing before an Administrative Law Judge, the company has evidently chosen to once again hide important health information from the Agency.
EWG reiterates a request we first made to EPA last August — that DuPont be fined the full amount of $313 million for its failure to disclose important health information. Unfortunately, even a penalty of this magnitude may barely be felt by a company whose profits last quarter exceeded that amount. When will DuPont begin to act responsibly?
The text from EWG's new petition to the EPA, detailing DuPont's suppression of important health information on their Teflon chemical, is given below.
November 17, 2004
Administrator Michael Leavitt
U.S. Environmental Protection Agency
Washington, D.C.
Re: DuPont's failure to submit a study of human blood pollution under the requirements of TSCA 8(e), 15 U.S.C. § 2607(e).
Dear Administrator Leavitt:
As you negotiate possible fines and penalties with DuPont for the company's illegal suppression of health and water pollution studies regarding its Teflon-related chemical, PFOA (perfluorooctanoic acid, or C8), we have learned of yet another, potential and very recent violation of Section 8(e) of the Toxic Substances Control Act, 15 U.S.C. § 2607(e).
Together with evidence compiled in our two prior submissions dated April 11 and August 15, 2003 (EWG 2003 a,b), the documentation we provide below suggests that DuPont is engaging in a continuing, flagrant pattern of suppressing information "that reasonably supports the conclusion that such substance...presents a substantial risk of injury to health" and that the company is required by law to report. 15 U.S.C § 2607(e).
In a study conducted through DuPont and its contractor Exygen dated July 29, 2004, DuPont learned of high levels of the Teflon chemical PFOA in serum from 12 people living near the company's Washington Works facility in Parkersburg, West Virginia (Exhibit A, B). The study shows that on average, Teflon chemical serum levels in this group — all of whom had consumed tap water contaminated with the Teflon chemical from DuPont's Washington Works operations and only one of whom had ever worked at the facility — are 12 times higher than levels measured previously from among the general population (67.5 ppb versus 5.6 ppb; EPA 2003; Exhibit A). DuPont found the Teflon chemical in one-quarter of the people tested at levels higher than have ever before been measured in the U.S. general population (Exhibit A, B; Kannan et al. 2004). The three highest levels were found in the serum of men and women who had consumed local tap water for more than 20 years.
This study clearly supports "the conclusion that such substance... presents a substantial risk of injury to health," given that the EPA's 2003 risk assessment had already documented Teflon chemical levels among female children and adult women as falling far outside of standard Agency safety margins (EPA 2003). How could the company have thought that this was not of immediate relevance to your Agency's decision making, and that it was not legally required to give you the study results?
In April 2003 EPA released a human health risk assessment showing that levels of PFOA measured in women's serum are as little as seven times lower than levels that showed no adverse effects in laboratory studies. This so-called "margin of exposure" — the ratio of the "no effect" levels from lab studies to human exposure levels — is well below the value of 100 typically required by the Agency for human health protection. DuPont's new study shows maximum levels in women far higher than those from previous studies (128 ppb versus 52.3 ppb; EPA 2003; Exhibit A), driving the margin of exposure down to a mere 2.9.
Results from DuPont's new study suggest that women of childbearing age in the Parkersburg area, consuming tap water contaminated with the Teflon chemical, are exposed far in excess of health-protective levels: Available data indicate that high levels of exposure to PFOA are more common among younger women and girls than among post-menopausal women like those included in DuPont's new study (Olsen et al., 2002a, 2002b, 2000c).
Under reporting deadlines established in 15 U.S.C. § 2607(e), DuPont was required to submit this study to EPA within 15 days of receipt. We find no 8(e) submission by DuPont in the Agency's 8(e) dockets. The study was submitted to EPA on September 15, 2004, not by DuPont, but by an outside law firm representing Parkersburg area residents who are served PFOA-contaminated tap water (Exhibit B). It appears that DuPont still has submitted no information on this study to EPA.
DuPont's new apparent reporting violation comes on the heels of your Agency's formal complaint against the company (EPA 2004), filed last July, in which you charged DuPont with three counts of failing to report company information on substantial health risks from other company studies regarding the Teflon chemical, as documented in EWG (2003 a,b). According to EPA's enforcement official, the complaint was filed with an intention "to send a message to DuPont and everyone else [that] this type of information must be provided" so officials "can make valid assessments of the risks posed by various substances" (Eilperin 2004). Although the charges the Agency made against DuPont carry a maximum penalty of $313 million, you failed to publicly recommend a penalty amount. As you know, the case now sits before an Administrative Law Judge. Clearly, DuPont failed to absorb the message you intended to send: despite EPA's formal complaint and pending hearing, DuPont continues its established pattern of hiding important health information from the Agency.
We reiterate a request we made on August 5 2004 (EWG 2004) — that you recommend the maximum possible fine of $313 million against DuPont for its previous failure to report critical information on Teflon chemical pollution of human blood and tap water. We also recommend that you consider the potential violation documented here, and again recommend the maximum possible penalty of $27,500 per day. At a time when your Agency is embroiled in one of the most significant human health risk assessments in its history, nothing short of the significant penalties we advocate will send the message to DuPont and other companies that you intended to send last July: companies like DuPont must submit information on significant health risks, in accordance with the law, to ensure that the Agency has full access to all information it needs to protect human health.
Sincerely,
[signed]
Kenneth A. Cook
President, Environmental Working Group
cc: Charles O. Holliday, Jr., Chairman & CEO, DuPont
Steve Johnson, Deputy Administrator, Environmental Protection Agency
References
Eilperin, Juliet. 2004. EPA to Fine DuPont for Silence on Teflon Chemical; Health Problems Alleged Near W. Va. Plant. The Washington Post. July 9, 2004.
Environmental Protection Agency. 2003. Preliminary Risk Assessment of the Developmental Toxicity Associated with Exposure to Perflourooctanoic Acid and its Salts [OPPT—2003—0012; FRL—7303—8]. April 10, 2003.
Environmental Protection Agency. 2004. Complaint and Notice of Opportunity for Hearing, Docket Nos. TSCA-HQ-2004-0016, RCRA-HQ-2004-0016. July 8, 2004.
Environmental Working Group. 2003a. Petition to Environmental Protection Agency. DuPont's failure to submit key health studies under the requirements of TSCA 8(e), 15 U.S.C. § 2607(e). April 11, 2003.
Environmental Working Group. 2003b. Supplement to Petition from Environmental Working Group to Environmental Protection Agency. DuPont's failure to submit key health studies under the requirements of TSCA 8(e), 15 U.S.C. § 2607(e). August 15, 2003.
Environmental Working Group. 2004. Letter from Timothy Kropp of EWG to Tom Skinner of Environmental Protection Agency. As you and your colleagues decide how much to fine DuPont... Available online at https://www.ewg.org/featured/228. Aug 5, 2004.
Kannan K, S Corsolini, J Falandysz, G Fillman, KS Kumar, BG Logananthan, MA Mohd, J Olivero, NV Wouwe, JH Yang, KM Aldous. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. & Technol. 38(17):4489-95.
Olsen GW, Burris JM, Lundberg JK, Hansen KJ, Mandel JH, Zobel LR. 2002a. Final Report: Identification of fluorochemicals in human sera. III. Pediatric participants in a group A streptococci clinical trial investigation U.S. EPA Administrative Record AR226-1085: Study conducted by Corporate Occupational Medicine, Medical Department, 3M Company, 220-3W-05, St. Paul, MN
Olsen GW, Burris JM, Lundberg JK, Hansen KJ, Mandel JH, Zobel LR. 2002b. Final Report: Identification of fluorochemicals in human sera. I. American Red Cross Adult Blood Donors U.S. EPA Administrative Record AR226-1083: Study conducted by Corporate Occupational Medicine, Medical Department, 3M Company, 220-3W-05, St. Paul, MN
Olsen GW, Burris JM, Lundberg JK, Hansen KJ, Mandel JH, Zobel LR. 2002c. Final Report: Identification of fluorochemicals in human sera. II. Elderly participants of the adult changes in thought study, Seattle, Washington U.S. EPA Administrative Record AR226-1083: Study conducted by Corporate Occupational Medicine, Medical Department, 3M Company, 220-3W-05, St. Paul, MN
Attachments
Exhibit A. Exygen Research. 2004. Analytical Report by Occupational and Environmental Health. Analysis of Perfluorooctanoic Acid (PFOA) in Human Serum Samples (Exygen Report No. L0003000). July 29, 2004.
Exhibit B. Bilott, R. 2004. Letter from Robert A. Bilott of Taft, Stettinium, & Hollister LLP to Dr. Charles M. Auer et al., Environmental Protection Agency. PFOA-Exposed Community Blood Sample Results (For AR-226 and OPPT-2003-0012). September 15, 2004.